Impurity Control Platform

Impurities refer to all substances that affect the purity of medicines. They not only affect the therapeutic effect of the drug, but also may be toxic. Impurity control is one of the most important requirements in drug quality management. BOC Sciences has developed various technologies to control impurities. We can control impurities by adding appropriate control measures in the process of process development and scale-up.
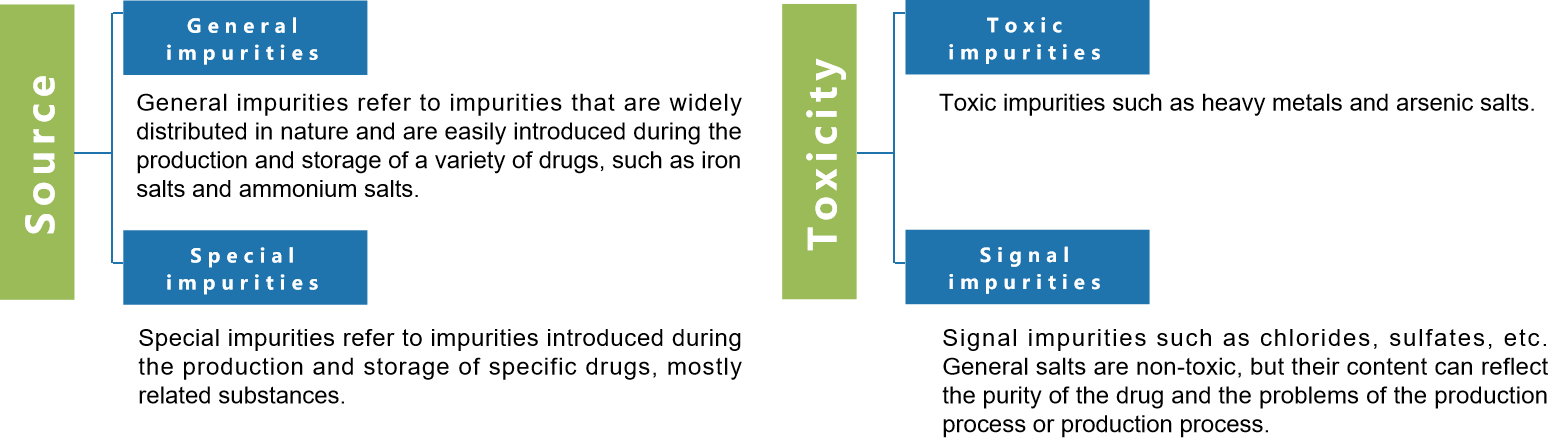
Classification of Impurities
According to the source, impurities can be divided into general impurities and special impurities; according to toxicity, impurities can be further divided into: toxic impurities and signal impurities.
Our Method
- Establishment and verification of impurity analysis methods: Comprehensively grasp the impurity profile of the product through impurity spectrum analysis, establish appropriate analysis methods for various impurities, and conduct systematic research and verification of the analysis methods to make the established analysis methods suitable for corresponding testing requirements.
- Impurity confirmation: Use established analytical methods to ensure the effective detection and confirmation of various impurities; track the impact of impurity profiles on the results of safety tests or clinical trials, and evaluate the acceptable levels of impurities in combination with relevant guidelines and literature information, establish impurity control limits for products.
- Source control: The source and quality of the raw materials are tested, and some impurities are eliminated from the source.
- Process control: Combining the actual production process and structural characteristics of the product, set up targeted control measures for corresponding impurities in the preparation process, and eliminate certain impurities before starting the process by controlling key steps and process parameters.
- Stability control: Study product packaging and storage conditions to effectively inhibit the degradation of drugs, so as to control impurities within a safe and reasonable range.
In order to control the clinical safety, quality controllability and product stability of drugs, BOC Sciences has established a systematic impurity research system. Our impurity control concept is established in accordance with the international guidelines for various impurity control, scientific and comprehensive.
If you are interested in our impurity control platform, please contact us immediately.