Heparins Custom Development
BOC Sciences can provide synthesis and manufacturing services for heparin and heparinoid drugs. Heparin drugs have significant effects in the treatment of thrombotic diseases, and are the only effective specific drugs in hemodialysis treatment. Heparin drugs are in rigid demand in the market, and with the expansion of clinical indications, their scale in the drug market is still gradually expanding. In addition to providing the production of heparin generic drugs, we also have strong R&D and innovation capabilities, which can help you launch new heparin APIs, so that you can always maintain your core competitiveness in the market.
What is Heparin?
Heparin is a mucopolysaccharide sulfate with the chemical formula C12H19NO20S3. It is a white or off-white crystalline powder. It is highly acidic, easily soluble in water, and insoluble in ethanol, chloroform and benzene. Heparin contains a large number of sulfate groups and negative charges, and its molecular weight is between 3,000 and 40,000. It is composed of glucosamine, L-iduronide, N-acetylglucosamine and D-glucuronic acid. Heparin is produced by mast cells and basophils, and is mainly found in lung, vascular wall, intestinal mucosa and other tissues. It is now mainly extracted from bovine lung or pig small intestinal mucosa. In addition, it is metabolized mainly in the liver to sulfate conjugates, which are inactive metabolites.
 Fig. 1. Preparation and application of heparin formulations (Carbohydrate Polymers. 2022, 295: 119818).
Fig. 1. Preparation and application of heparin formulations (Carbohydrate Polymers. 2022, 295: 119818).
Whether in vivo or in vitro, heparin has a strong anticoagulant effect, mainly by enhancing the activity of antithrombin and inhibiting the activity of thrombin and other coagulation factors. Heparin also has various biological activities such as inhibiting platelet aggregation, regulating blood lipids, anti-inflammatory, anti-allergic, and anti-cancer. Clinically, it is mainly used to prevent and treat various thromboembolic diseases, disseminated intravascular coagulation, myocardial infarction, etc., and to maintain blood fluidity during cardiovascular surgery, extracorporeal circulation, hemodialysis, etc.
Types of Heparin
Since the discovery of heparin, it has been widely used to prevent and treat various thromboembolic diseases due to its rapid onset of action, definite efficacy, and reversible anticoagulant effect. At present, heparin is mainly divided into unfractionated heparin (UFH), low molecular weight heparin (LMWH), heparin derivatives (such as fondaparinux), and heparin analogs (such as danaparinux).
- Unfractionated heparin is a mixture of sulfated glycosaminoglycans (GAGs), a mucopolysaccharide sulfate consisting of D-glucosamine, L-iduronic acid and D-glucuronic acid alternately. It can be prepared from the lungs of cattle or the intestinal mucosa of cattle, sheep, and pigs.
- Low molecular weight heparin is a short-chain preparation obtained from the separation of ordinary heparin or the degradation of ordinary heparin. Due to the differences in molecular size, anticoagulant activity, preparation methods, manufacturers, etc., low molecular weight heparin commonly used in clinical practice includes enoxaparin, dalteparin, nadroparin, etc.
- Synthetic heparin derivative fondaparinux sodium is a pentopolysaccharide synthesized based on the structure of antithrombin heparin binding site.
- Heparin analogue danaparin sodium is a sulfated aminoglucan mixture. It was also prepared from pig intestinal mucosa, and the main components were heparan sulfate, dermatan sulfate and chondroitin sulfate.
Heparin Drug Development Services
BOC Sciences is a leading provider of custom heparin development services, offering a range of solutions for the development and production of heparin and related compounds. We offer comprehensive heparin generic and brand-name support services designed to meet our customers' unique needs.
Heparin Extraction Services
Heparin is usually produced by the mucosal tissue of pigs or cattle. The process involves extracting heparin from tissue, purifying it and then formulating it into a pharmaceutical product such as an injection or ointment. Extraction and purification processes typically involve several steps, including chemical and enzymatic treatments, as well as filtration and chromatography. The final product is then tested for purity and potency, followed by drug research and development. BOC Sciences has extensive experience in the extraction of heparin and related compounds. We can develop custom strategies to produce heparin in large quantities with high purity and consistency.
Heparin Analysis Services
BOC Sciences has state-of-the-art capabilities for the analysis of heparin and related compounds. We can perform a range of tests, including structural analysis, purity testing and impurity analysis, to ensure the quality and safety of the final product.
Heparin Modification Services
BOC Sciences can modify the structure of heparin to create new derivatives with specific properties. This can include adding functional groups, modifying molecular weight, or developing new formulations.
Heparin Preparations
BOC Sciences can develop custom heparin formulations based on customers' specific needs. This can include developing new drug delivery systems, such as nanoparticles or liposomes, or optimizing existing formulations.
Heparin Amplification Services
BOC Sciences has the capability to scale up the production of heparin and related compounds from laboratory to commercial scale. We can develop custom manufacturing processes to ensure efficient and cost-effective production of heparin.
Our Low Molecular Weight Heparin Preparations
| Preparation | Method of Preparation | Molecular Weight |
| Ardeparin | Peroxidative depolymerisation | 6000 |
| Dalteparin | Nitrous acid depolymerisation | 6000 |
| Enoxaparin | Alkaline depolymerisation | 4200 |
| Nadroparin | Nitrons acid depolymerisation | 4500 |
| Reviparin | Nitrous acid depolymerisation | 4000 |
| Tinzaparin | Heparinase digestion | 4500 |
Heparin Manufacturing Capacities
BOC Sciences has a team of highly skilled scientists and engineers with expertise in the development and production of heparin and related compounds. We have an in-depth understanding of the chemical and biological properties of heparin and the regulatory requirements for its development and production.
Technology development: Utilize chemical and fusion protein technology to customize heparin preparation methods to efficiently produce high-purity heparin and heparoids.
Process exploration and optimization: Explore and optimize key production processes through single-factor experiments, multi-factor combination experiments, response surface data analysis and other means to obtain the best heparin process parameters. On this basis, it was confirmed through experiments that the optimized production process can produce heparin products with stable quality.
Quality assurance: State-of-the-art heparin extraction, analysis and formulation facilities, including high-performance liquid chromatography (HPLC), mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy, to ensure product effectiveness and uniformity.
Quality control: Starting from the heparin quality standard research method, and based on key indicators such as heparin molecular weight, amino sugars, sulfates, and structure, a modern instrument analysis method for heparin quality control research is established. This makes heparin quality control more comprehensive, thereby improving the quality, safety and reliability of heparin drugs.
Why Choose BOC Sciences?
- Expert drug development knowledge and project experience: BOC Sciences has a highly qualified team of scientists and engineers with extensive expertise in the development and production of heparin. We can provide valuable insight and guidance throughout the development process, from initial concept to final product.
- Consistency of quality and safety: BOC Sciences has state-of-the-art heparin extraction, analysis and formulation facilities. We usually ensure the quality and safety of our products through strict testing and quality control processes.
- One-stop customized solutions: BOC Sciences can develop customized solutions for the development and production of heparin based on customers' specific needs, including developing new formulations, modifying molecular structures, or scaling up production processes.
- Regulatory compliance: We have an in-depth understanding of the regulatory requirements for heparin development and manufacturing. We can ensure products comply with relevant regulatory standards, including current Good Manufacturing Practice (GMP) and International Conference on Harmonization (ICH) guidelines.
- Cost-effective service guidelines: BOC Sciences can develop cost-effective solutions for the development and production of heparin, ensuring customers can achieve their goals within budget constraints.
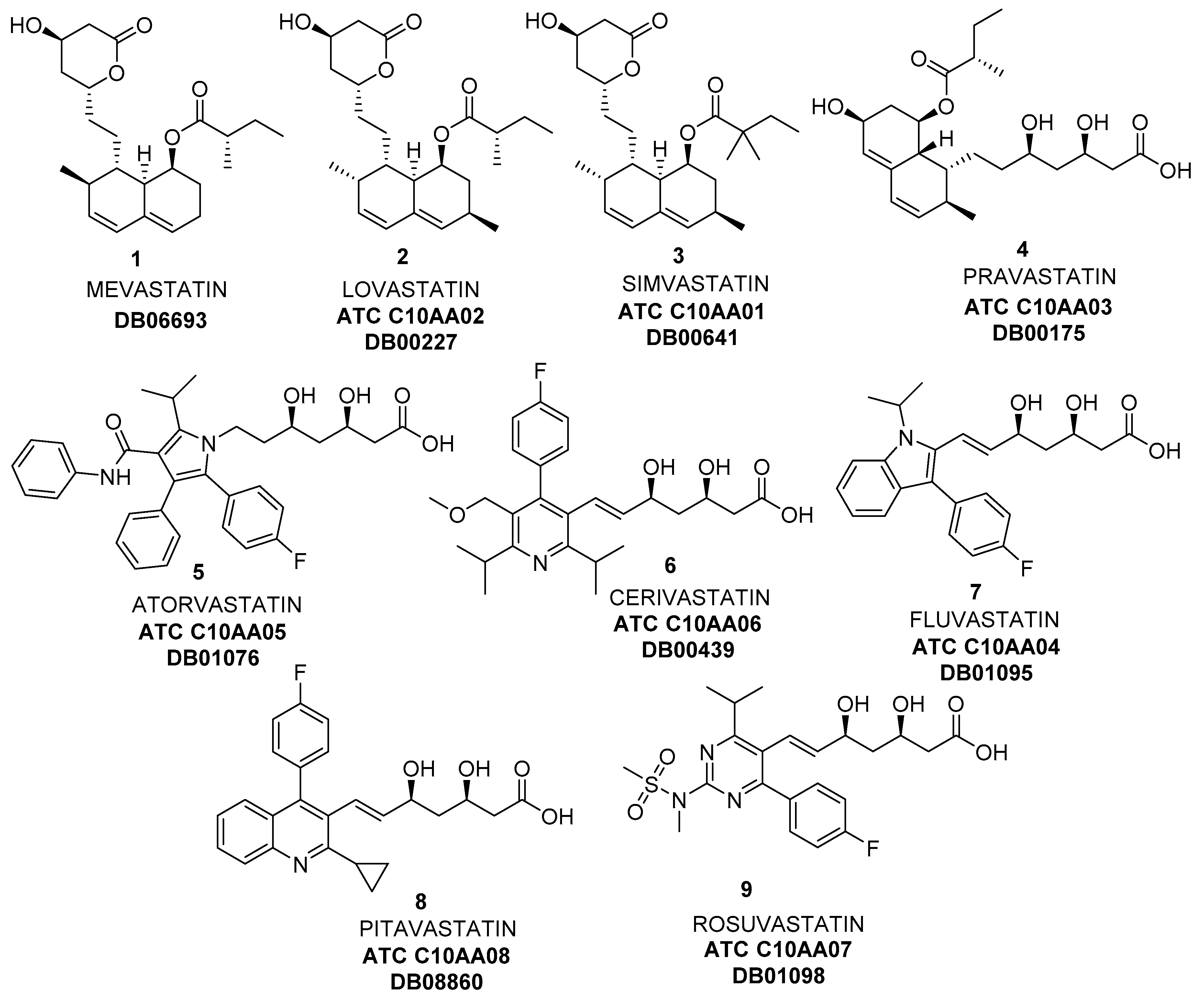
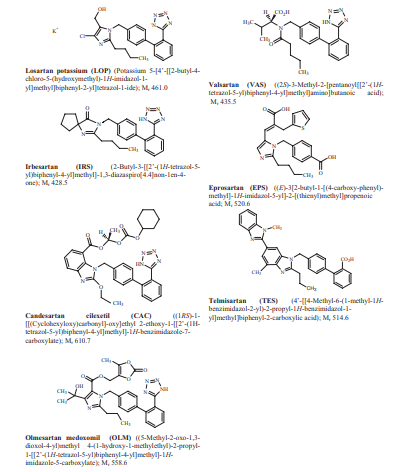
In addition to the custom development of heparin, we also provide custom development of other specialty APIs and intermediates, including angiotensin receptor blockers (ARB), hydroxymethylglutaryl-CoA reductase inhibitors (statins), psychiatry and neurology APIs, tyrosine kinase inhibitors (TKIs), contrast media, etc. If you are interested in our heparins custom development service, please contact us immediately.
Reference
- Wang, P. et al. Heparin: An old drug for new clinical applications. Carbohydrate Polymers. 2022, 295: 119818.